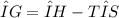Which of the following is true?
a. a reaction in which the entropy of the system increases can be spontaneous only if it is endothermic.
b. a reaction in which the entropy of the system decreases can be spontaneous only if it is endothermic.
c. a reaction in which the entropy of the system decreases can be spontaneous only if it is exothermic.
d. a reaction in which the entropy of the system increases can be spontaneous only if it is exothermic.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

You know the right answer?
Which of the following is true?
a. a reaction in which the entropy of the system increases ca...
a. a reaction in which the entropy of the system increases ca...
Questions

Mathematics, 19.05.2021 17:40

History, 19.05.2021 17:40



Mathematics, 19.05.2021 17:40

Mathematics, 19.05.2021 17:40

Mathematics, 19.05.2021 17:40

Computers and Technology, 19.05.2021 17:40



Mathematics, 19.05.2021 17:40




Mathematics, 19.05.2021 17:40


English, 19.05.2021 17:40


Mathematics, 19.05.2021 17:40

Chemistry, 19.05.2021 17:40