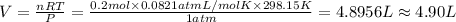Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
When aluminum is placed in concentrated hydrochloric acid, hydrogen gas is produced. 2 al ( s ) + 6...
Questions

Mathematics, 13.08.2021 14:00


Mathematics, 13.08.2021 14:00

Chemistry, 13.08.2021 14:00


English, 13.08.2021 14:00



Biology, 13.08.2021 14:00




Mathematics, 13.08.2021 14:00

Mathematics, 13.08.2021 14:00


Chemistry, 13.08.2021 14:00

Advanced Placement (AP), 13.08.2021 14:00

English, 13.08.2021 14:00


Biology, 13.08.2021 14:00

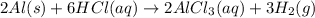
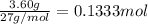
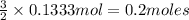 of hydrogen gas
of hydrogen gas