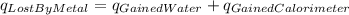In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used to determine the specific heat of a solid, or to measure the energy of a solution phase reaction. a student heats 61.68 grams of gold to 99.01 °c and then drops it into a cup containing 79.34 grams of water at 22.14 °c. she measures the final temperature to be 23.98 °c. the heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.80 j/°c. assuming that no heat is lost to the surroundings calculate the specific heat of gold.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

You know the right answer?
In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used t...
Questions




History, 26.11.2021 23:30


SAT, 26.11.2021 23:30



History, 26.11.2021 23:30

Mathematics, 26.11.2021 23:30



SAT, 26.11.2021 23:30

Health, 26.11.2021 23:30

History, 26.11.2021 23:30

Biology, 26.11.2021 23:30


Mathematics, 26.11.2021 23:30

History, 26.11.2021 23:30

Biology, 26.11.2021 23:30