If you are given an ideal gas with pressure (p) = 259,392.00 pa and temperature (t) = 2.00 oc
...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
Questions

Biology, 05.01.2020 23:31

Mathematics, 05.01.2020 23:31

Mathematics, 05.01.2020 23:31

Chemistry, 05.01.2020 23:31


Mathematics, 05.01.2020 23:31




History, 05.01.2020 23:31


Mathematics, 05.01.2020 23:31




Mathematics, 05.01.2020 23:31

Mathematics, 05.01.2020 23:31

Spanish, 05.01.2020 23:31


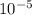 atm
atm

