
Chemistry, 28.11.2019 21:31 mervindavisk
Which of the following statements about the kinetic molecular theory are not correct?
a. the combined volume of all the molecules of the gas is negligible relative to the total volume in which the gas is contained
b. gases consist of molecules that are in continuous, random motion
c. the average kinetic energy of the molecules is not related to the absolute temperature
d. attractive and repulsive forces between gas molecules are negligible

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1

Chemistry, 23.06.2019 09:30
What is the best describtion of the side of the moon that faces earth?
Answers: 1

Chemistry, 23.06.2019 17:50
What is a property of a substance that is composed of atoms that are held together by ionic bonds?
Answers: 3
You know the right answer?
Which of the following statements about the kinetic molecular theory are not correct?
a. the...
a. the...
Questions



Mathematics, 19.03.2020 05:48

History, 19.03.2020 05:48

Physics, 19.03.2020 05:48

English, 19.03.2020 05:48

Mathematics, 19.03.2020 05:48







Physics, 19.03.2020 05:49

Computers and Technology, 19.03.2020 05:50



Chemistry, 19.03.2020 05:51


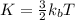
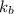 is the Boltzmann constant
is the Boltzmann constant

