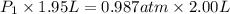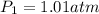Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 23.06.2019 08:30
Plz a person walks 1 mile every day for exercise, leaving her front porch at 9 am and returning to her front porch at 9: 25 am what was the total displacement of her daily walk a. 1 mile b. 0 c. 25 min d. none of the above
Answers: 2
You know the right answer?
A2.00-l sample of was collected over water at a total pressure of 750. torr and 22 °c. when the was...
Questions

Biology, 24.08.2019 10:10



History, 24.08.2019 10:10

Social Studies, 24.08.2019 10:10

Mathematics, 24.08.2019 10:10

Spanish, 24.08.2019 10:10


Chemistry, 24.08.2019 10:10

Business, 24.08.2019 10:10

English, 24.08.2019 10:10

Biology, 24.08.2019 10:10




History, 24.08.2019 10:20

Chemistry, 24.08.2019 10:20

Social Studies, 24.08.2019 10:20


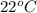 is 1.01 atm.
is 1.01 atm.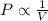
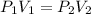
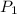 = initial pressure = ?
= initial pressure = ?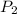 = final pressure = 750 torr = 0.987 atm (1 atm = 760 torr)
= final pressure = 750 torr = 0.987 atm (1 atm = 760 torr)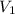 = initial volume = 1.95 L
= initial volume = 1.95 L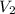 = final volume = 2.00 L
= final volume = 2.00 L