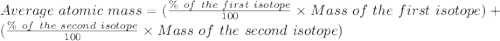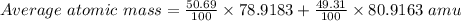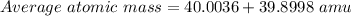Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

You know the right answer?
Bromine has two isotopes 79br and 81br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69...
Questions



Mathematics, 19.01.2021 19:10





English, 19.01.2021 19:10

Mathematics, 19.01.2021 19:10






Social Studies, 19.01.2021 19:10


Mathematics, 19.01.2021 19:10

Mathematics, 19.01.2021 19:10

Geography, 19.01.2021 19:10