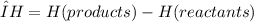Chemistry, 29.11.2019 00:31 foralways3348
An endothermic reaction has an endothermic reaction has a positive δh, absorbs heat from the surroundings, and feels cold to the touch. a positive δh, absorbs heat from the surroundings, and feels warm to the touch. a positive δh, gives off heat to the surroundings, and feels warm to the touch. a negative δh, absorbs heat from the surroundings, and feels cold to the touch. a negative δh, gives off heat to the surroundings, and feels warm to the touch.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
An endothermic reaction has an endothermic reaction has a positive δh, absorbs heat from the surroun...
Questions



English, 11.10.2020 03:01

Mathematics, 11.10.2020 03:01

Mathematics, 11.10.2020 03:01

Mathematics, 11.10.2020 03:01

Mathematics, 11.10.2020 03:01

English, 11.10.2020 03:01


Chemistry, 11.10.2020 03:01


History, 11.10.2020 03:01

Mathematics, 11.10.2020 03:01

Chemistry, 11.10.2020 03:01

Medicine, 11.10.2020 03:01


Mathematics, 11.10.2020 03:01


Geography, 11.10.2020 03:01