
Chemistry, 29.11.2019 02:31 mathman783
Given that δ h ∘ f [ br ( g ) ] = 111.9 kj ⋅ mol − 1 δ h ∘ f [ c ( g ) ] = 716.7 kj ⋅ mol − 1 δ h ∘ f [ cbr 4 ( g ) ] = 29.4 kj ⋅ mol − 1 calculate the average molar bond enthalpy of the carbon‑bromine bond in a cbr 4 molecule.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1


Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
Given that δ h ∘ f [ br ( g ) ] = 111.9 kj ⋅ mol − 1 δ h ∘ f [ c ( g ) ] = 716.7 kj ⋅ mol − 1 δ h ∘...
Questions

English, 20.08.2019 13:30

Biology, 20.08.2019 13:30


History, 20.08.2019 13:30



English, 20.08.2019 13:30

Social Studies, 20.08.2019 13:30


Mathematics, 20.08.2019 13:30


Mathematics, 20.08.2019 13:30

History, 20.08.2019 13:30


Mathematics, 20.08.2019 13:30


Mathematics, 20.08.2019 13:30


Social Studies, 20.08.2019 13:30

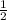 Br2(g) ⇒ Br(g) , Δ H ∘ = 111.9 kJ ⋅ mol − 1
Br2(g) ⇒ Br(g) , Δ H ∘ = 111.9 kJ ⋅ mol − 1 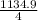 = 283.725 kJ ⋅ mol − 1
= 283.725 kJ ⋅ mol − 1

