Chemistry, 29.11.2019 03:31 jordanmazer17
Enter your answer in the provided box. s(rhombic) + o2(g) → so2(g) δho rxn= −296.06 kj/mols(monoclinic) + o2(g) → so2(g) δho rxn= −296.36 kj/molcalculate the enthalpy change for the transformations(rhombic) → s(monoclinic)(monoclinic and rhombic are different allotropic forms of elemental /mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
Enter your answer in the provided box. s(rhombic) + o2(g) → so2(g) δho rxn= −296.06 kj/mols(monoclin...
Questions

Spanish, 20.05.2020 22:07

Mathematics, 20.05.2020 22:07


History, 20.05.2020 22:07

Mathematics, 20.05.2020 22:07






Mathematics, 20.05.2020 22:07



Mathematics, 20.05.2020 22:07

History, 20.05.2020 22:07

Mathematics, 20.05.2020 22:07

Health, 20.05.2020 22:07

Mathematics, 20.05.2020 22:07

Mathematics, 20.05.2020 22:07

History, 20.05.2020 22:07

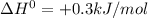 .
.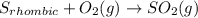
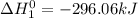 (1)
(1)
![S_{monoclinic}+O_2(g)\rightarrow SO_2(g)/tex] [tex]\Delta H^0_2=-296.36kJ](/tpl/images/0395/6241/b4124.png) (2)
(2)
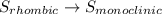
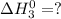 (3)
(3)