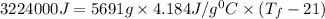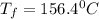The balanced combustion reaction for c 6 h 6 is 2 c 6 h 6 ( l ) 15 o 2 ( g ) ⟶ 12 co 2 ( g ) 6 h 2 o ( l ) 6542 kj if 7.700 g c 6 h 6 is burned and the heat produced from the burning is added to 5691 g of water at 21 ∘ c, what is the final temperature of the water

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
The balanced combustion reaction for c 6 h 6 is 2 c 6 h 6 ( l ) 15 o 2 ( g ) ⟶ 12 co 2 ( g ) 6 h 2 o...
Questions

Chemistry, 26.08.2019 07:30

History, 26.08.2019 07:30





Geography, 26.08.2019 07:30

Social Studies, 26.08.2019 07:30




History, 26.08.2019 07:30


Physics, 26.08.2019 07:30

Mathematics, 26.08.2019 07:30

Biology, 26.08.2019 07:30

English, 26.08.2019 07:30



Chemistry, 26.08.2019 07:30

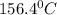
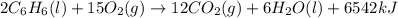
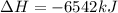
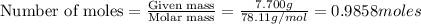
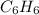 releases = 6542 kJ of heat
releases = 6542 kJ of heat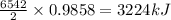 of heat
of heat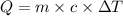
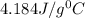
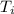 = 21.0°C
= 21.0°C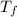 = ?
= ?