Chemistry, 29.11.2019 05:31 SophieCasey
Asilver-copper alloy had a mass of 0.1264g. when the alloy was dissolved in nitric acid and the silver precipitated as silver chloride, the precipitate had a mass of 0.1375g. calculate the percent of silver in the alloy. for full credit, make sure you show your calculations.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1


Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
Asilver-copper alloy had a mass of 0.1264g. when the alloy was dissolved in nitric acid and the silv...
Questions







Biology, 25.10.2019 04:43













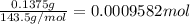
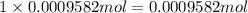 of silver
of silver