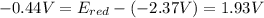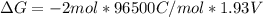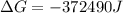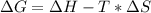Chemistry, 29.11.2019 06:31 Supermate11
Free-energy change, δg∘, is related to cell potential, e∘, by the equationδg∘=−nfe∘where n is the number of moles of electrons transferred and f=96,500c/(mol e−) is the faraday constant. when e∘ is measured in volts, δg∘ must be in joules since 1 j=1 c⋅v.1. calculate the standard free-energy change at 25 ∘c for the following reaction: mg(s)+fe2+(aq)→mg2+(aq)+fe(s)expres s your answer to three significant figures and include the appropriate units.2. calculate the standard cell potential at 25 ∘c for the reactionx(s)+2y+(aq)→x2+(aq)+2y(s)w here δh∘ = -675 kj and δs∘ = -357 j/k .express your answer to three significant figures and include the appropriate units.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2


Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
Free-energy change, δg∘, is related to cell potential, e∘, by the equationδg∘=−nfe∘where n is the nu...
Questions

English, 11.10.2019 15:00



Biology, 11.10.2019 15:00

Mathematics, 11.10.2019 15:00




Mathematics, 11.10.2019 15:00

Mathematics, 11.10.2019 15:00

Biology, 11.10.2019 15:00

Mathematics, 11.10.2019 15:00


Physics, 11.10.2019 15:00



Mathematics, 11.10.2019 15:00

History, 11.10.2019 15:00


Mathematics, 11.10.2019 15:00

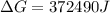
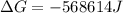
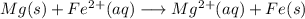
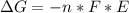
![E=E_{red}-E_{ox]](/tpl/images/0395/8514/34231.png)