
Sulfur dioxide, so2(g), can react with oxygen to produce sulfur trioxide, so3(g), by the following reaction
2so2+o2=2so3
the standard enthalpies of formation for so2(g) and so3(g) are
deltah= so2(g)= -296.8 kj
dh= so3(g)= -395.7 kj
calculate the amount of energy in the form of heat that is produced when a volume of 3.75 l of so2(g) is converted to 3.75 l of so3(g) according to this process at a constant pressure and temperature of 1.00 atm and 25.0

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

You know the right answer?
Sulfur dioxide, so2(g), can react with oxygen to produce sulfur trioxide, so3(g), by the following r...
Questions


Mathematics, 07.04.2020 20:29

Computers and Technology, 07.04.2020 20:29

English, 07.04.2020 20:29


History, 07.04.2020 20:29

Chemistry, 07.04.2020 20:29



Chemistry, 07.04.2020 20:30


Mathematics, 07.04.2020 20:30


Biology, 07.04.2020 20:30


History, 07.04.2020 20:30

Mathematics, 07.04.2020 20:30


Mathematics, 07.04.2020 20:30

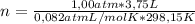 = 0,153 moles of reaction
= 0,153 moles of reaction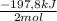 = -15,1kJ
= -15,1kJ


