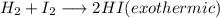Chemistry, 29.11.2019 07:31 heathkid23
 + i_2(g) \longrightarrow 2hi(g))
the forward reaction above is exothermic. at equilibrium, what happens if the reaction mixture is cooled at constant volume?
select all that apply.
1. the reaction absorbs energy.
2. the reaction releases energy.
3. [h₂] and [i₂] increase.
4. [h₂] and [i₂] decrease.
5. [h₂] and [i₂] remain constant.
6. [hi] increases.
7. [hi] decreases.
8. [hi] remains constant.
if c is added to the equilibrium system above, in which direction will the equilibrium shift?

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
[tex]h_2(g) + i_2(g) \longrightarrow 2hi(g)[/tex]
the forward reaction above is exothermic. at...
the forward reaction above is exothermic. at...
Questions

Mathematics, 08.10.2019 23:40


Mathematics, 08.10.2019 23:40



History, 08.10.2019 23:40

Mathematics, 08.10.2019 23:40

Geography, 08.10.2019 23:40

Mathematics, 08.10.2019 23:40



Biology, 08.10.2019 23:40


Mathematics, 08.10.2019 23:40


Geography, 08.10.2019 23:40

English, 08.10.2019 23:40

Mathematics, 08.10.2019 23:40

Mathematics, 08.10.2019 23:40

![[H_{2}]](/tpl/images/0395/9023/f6f78.png) and
and ![[I_{2}]](/tpl/images/0395/9023/d0626.png) increase.
increase.![[HI]](/tpl/images/0395/9023/7d1e5.png) increases.
increases.