
Chemistry, 30.11.2019 00:31 mariposa91
Calculate the grams of so2 gas present at stp in a 5.9 l container. (r = 0.0821 l·atm/k·mol)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2


Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
Calculate the grams of so2 gas present at stp in a 5.9 l container. (r = 0.0821 l·atm/k·mol)...
Questions



Social Studies, 21.07.2020 20:01


Mathematics, 21.07.2020 20:01





Mathematics, 21.07.2020 20:01




Chemistry, 21.07.2020 20:01


Computers and Technology, 21.07.2020 20:01

Mathematics, 21.07.2020 20:01



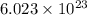 of particles.
of particles.
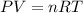
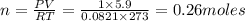
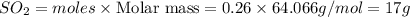
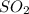 gas is present at STP in a 5.9 L container.
gas is present at STP in a 5.9 L container.

