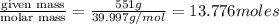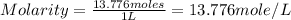Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Sodium hydroxide is extremely soluble in water. at a certain temperature, a saturated solution conta...
Questions

Mathematics, 19.07.2019 03:30

History, 19.07.2019 03:30




Biology, 19.07.2019 03:30

Geography, 19.07.2019 03:30


English, 19.07.2019 03:30

Social Studies, 19.07.2019 03:30




English, 19.07.2019 03:30





Mathematics, 19.07.2019 03:30

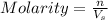
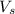 = volume of solution in L = 1 L
= volume of solution in L = 1 L