
Chemistry, 30.11.2019 00:31 StacySams7361
Achemist combined chloroform ( chcl 3 ) and acetone ( c 3 h 6 o ) to create a solution where the mole fraction of chloroform, χ chloroform , is 0.203 . the densities of chloroform and acetone are 1.48 g/ml and 0.791 g/ml , respectively. calculate the molarity of the solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1


Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1
You know the right answer?
Achemist combined chloroform ( chcl 3 ) and acetone ( c 3 h 6 o ) to create a solution where the mol...
Questions

History, 26.08.2020 19:01


History, 26.08.2020 19:01

Biology, 26.08.2020 19:01



Mathematics, 26.08.2020 19:01





Mathematics, 26.08.2020 19:01




Mathematics, 26.08.2020 19:01

Mathematics, 26.08.2020 19:01



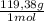 ×
×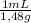 = 1637mL= 1,637L
= 1637mL= 1,637L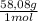 ×
×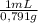 = 5852mL = 5,852L
= 5852mL = 5,852L

