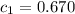Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2
You know the right answer?
When a 40.0 g sample of a metal at 25.00 °c is added to 65.0 g of water at 100.00 °c, the final temp...
Questions



Biology, 19.10.2019 00:00

Mathematics, 19.10.2019 00:00




Social Studies, 19.10.2019 00:00

Physics, 19.10.2019 00:00

Mathematics, 19.10.2019 00:00

Biology, 19.10.2019 00:00

Mathematics, 19.10.2019 00:00


Chemistry, 19.10.2019 00:00


Mathematics, 19.10.2019 00:00


History, 19.10.2019 00:00


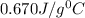
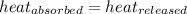
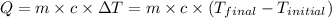
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0396/4522/09236.png) .................(1)
.................(1)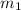 = mass of metal = 40.0 g
= mass of metal = 40.0 g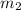 = mass of water = 65.0 g
= mass of water = 65.0 g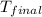 = final temperature =
= final temperature = 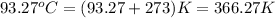
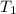 = temperature of metal =
= temperature of metal = 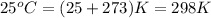
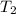 = temperature of water =
= temperature of water = 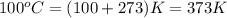
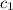 = specific heat of metal = ?
= specific heat of metal = ?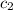 = specific heat of water=
= specific heat of water= 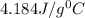
![40.0\times c_1\times (366.27-298)=-[65.0\times 4.184\times (366.27-373)]](/tpl/images/0396/4522/88e12.png)