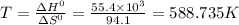Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3


Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
You know the right answer?
For a particular reaction, δ h ∘ = 55.4 kj δh∘=55.4 kj and δ s ∘ = 94.1 j/k. δs∘=94.1 j/k. assuming...
Questions

Mathematics, 19.10.2021 21:50


Mathematics, 19.10.2021 21:50



Mathematics, 19.10.2021 21:50

SAT, 19.10.2021 21:50

Mathematics, 19.10.2021 21:50



Mathematics, 19.10.2021 21:50

Chemistry, 19.10.2021 21:50



Chemistry, 19.10.2021 21:50


Computers and Technology, 19.10.2021 21:50



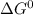 .
.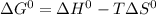
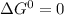 as the limiting condition.
as the limiting condition.