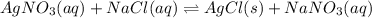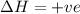Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
For the endothermic reaction below, using lechatlier's principle, predict which way the equilibrium...
Questions


Mathematics, 02.12.2021 05:20

SAT, 02.12.2021 05:20

Mathematics, 02.12.2021 05:20

Social Studies, 02.12.2021 05:20


Social Studies, 02.12.2021 05:20



Chemistry, 02.12.2021 05:20


Mathematics, 02.12.2021 05:20

Mathematics, 02.12.2021 05:20


Mathematics, 02.12.2021 05:20



Biology, 02.12.2021 05:20


Mathematics, 02.12.2021 05:20