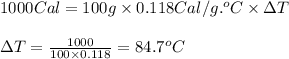The specific heats and densities of several materials are given below:
material specific heat...

The specific heats and densities of several materials are given below:
material specific heat (cal/g·°c) density (g/cm3)
brick 0.220 2.0
concrete 0.270 2.7
steel 0.118 7
water 1.00 1.00
calculate the change in temperature produced by the addition of 1 kcal of heat to 100 g of steel.
a. 84.7°c
b. 37.0°c
c. 1.43°c
d. 1.18°c

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Questions

English, 21.08.2019 22:10

English, 21.08.2019 22:10


Health, 21.08.2019 22:10






Biology, 21.08.2019 22:10




Physics, 21.08.2019 22:10



Computers and Technology, 21.08.2019 22:10



Mathematics, 21.08.2019 22:10

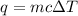
 = change in temperature = ?
= change in temperature = ?