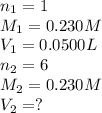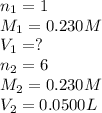For the following reaction between mohr's salt (iron as feso_4(nh_4)_2so_4 6h_2o) and potassium dichromate (dichromate as k_2cr_2o_7), determine the volume (in milliliters) of a 0.230 m solution of mohr's salt that is needed to fully react with 0.0500 l of 0.230 m potassium dichromate. (the reaction is shown in its ionic form in the presence of a strong acid.)
cr_2o_7^2- + 6fe^2+ + 14h^+ rightarrow 2cr^3+ + 6fe^3+ + 7h_2o
mohr's salt volume = ml
for the same reaction, what volume (in milliliters) of 0.230 m potassium dichromate is required to fully react with 0.0500 l of a 0.230 m solution of mohr's salt
potassium dichromate volume = ml

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 05:30
Suppose you discovered a new element with 120 protons and 2 electrons in its outer level . i'm what group does this new element belong? what properties would you expect it to have
Answers: 1
You know the right answer?
For the following reaction between mohr's salt (iron as feso_4(nh_4)_2so_4 6h_2o) and potassium dich...
Questions




Mathematics, 07.04.2020 15:28






History, 07.04.2020 15:28

Mathematics, 07.04.2020 15:28







Mathematics, 07.04.2020 15:29


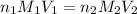
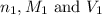 are the n-factor, molarity and volume of
are the n-factor, molarity and volume of 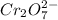
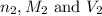 are the n-factor, molarity and volume of
are the n-factor, molarity and volume of ![Fe^{2+]](/tpl/images/0396/5656/ad855.png)