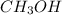Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: co(g)+2h2(g)→ch3oh(g) a 1.65 l reaction vessel, initially at 305 k, contains carbon monoxide gas at a partial pressure of 232 mmhg and hydrogen gas at a partial pressure of 374 mmhg .identify the limiting reactant and determine the theoretical yeild of methonal in grams.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1


Chemistry, 23.06.2019 06:10
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1
You know the right answer?
Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: co(g)+2h2...
Questions



Mathematics, 18.02.2020 18:25

English, 18.02.2020 18:25




Mathematics, 18.02.2020 18:25




English, 18.02.2020 18:25



Physics, 18.02.2020 18:25



Computers and Technology, 18.02.2020 18:25


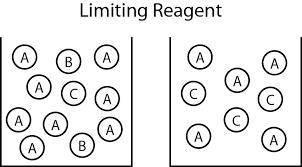
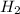 and the theoretical yield of methanol is, 0.96 grams.
and the theoretical yield of methanol is, 0.96 grams. and
and 
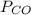 = pressure of CO gas = 232 mmHg = 0.305 atm (1 atm = 760 mmHg)
= pressure of CO gas = 232 mmHg = 0.305 atm (1 atm = 760 mmHg)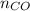 = number of moles of CO gas = ?
= number of moles of CO gas = ?
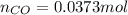

 = pressure of
= pressure of  = number of moles of
= number of moles of 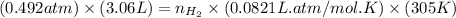


 moles of
moles of