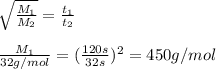Chemistry, 30.11.2019 01:31 hannahbeccahxo9681
Agas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure conditions. it required 120 s for 1.0 l of the gas to effuse. under identical experimental conditions it required 32 s for 1.0 l of o2 gas to effuse. you may want to reference (pages 416 - 419) section 10.8 while completing this problem. part a calculate the molar mass of the unknown gas. (remember that the faster the rate of effusion, the shorter the time required for effusion of 1.0 l; that is, rate and time are inversely proportional.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1
You know the right answer?
Agas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure...
Questions



Biology, 22.12.2021 05:50



Computers and Technology, 22.12.2021 06:00


Business, 22.12.2021 06:00







SAT, 22.12.2021 06:00