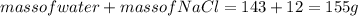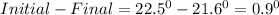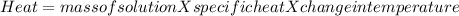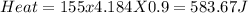1. a calorimeter contains 143 g of water at 22.5°c. a 12 g sample of nacl is added to the water in the calorimeter. after the solid has dissolved, the temperature of the water is 21.6°c. calculate the enthalpy of solution for dissolving sodium chloride. assume that no heat is lost to the calorimeter or the surroundings and that the specific heat of the solution is the same as that of pure water.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2
You know the right answer?
1. a calorimeter contains 143 g of water at 22.5°c. a 12 g sample of nacl is added to the water in t...
Questions


Chemistry, 21.01.2021 22:10

History, 21.01.2021 22:10

English, 21.01.2021 22:10


Mathematics, 21.01.2021 22:10






History, 21.01.2021 22:10





Mathematics, 21.01.2021 22:10



Physics, 21.01.2021 22:10