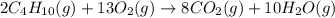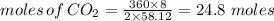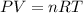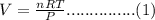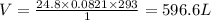Chemistry, 30.11.2019 02:31 mbatton879
Combustion of hydrocarbons such as butane () produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.1. write a balanced chemical equation, including physical state symbols, for the combustion of gaseous butane into gaseous carbon dioxide and gaseous water.2. suppose 0.360 kg of butane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 °c. calculate the volume of carbon dioxide gas that is produced be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Combustion of hydrocarbons such as butane () produces carbon dioxide, a "greenhouse gas." greenhouse...
Questions

Computers and Technology, 26.05.2021 17:10



Mathematics, 26.05.2021 17:10

Mathematics, 26.05.2021 17:10




English, 26.05.2021 17:10


Biology, 26.05.2021 17:10



Mathematics, 26.05.2021 17:10




Mathematics, 26.05.2021 17:10

Mathematics, 26.05.2021 17:10