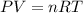Chemistry, 30.11.2019 02:31 megamegs80
Calculate the volume of the gas when the pressure of the gas is 1.60 atm at a temperature of 298 k. there are 160. mol of gas in the cylinder. the value for the universal gas constant r is 0.08206 l⋅atm/(mol⋅k) .
express your answer numerically to four significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
Calculate the volume of the gas when the pressure of the gas is 1.60 atm at a temperature of 298 k....
Questions



Mathematics, 06.11.2020 19:30



History, 06.11.2020 19:30

Mathematics, 06.11.2020 19:30


English, 06.11.2020 19:30




Physics, 06.11.2020 19:30

English, 06.11.2020 19:30



Mathematics, 06.11.2020 19:30