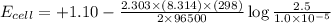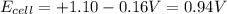The standard cell potential (e°cell) for the reaction below is +1.10v. the cell potential for this reaction is v when the concentration of [cu2+]=1.0⋅10−5m and [zn2+]=2.5m. zn (s) + cu2+ (aq) → cu (s) + zn2+ (aq) the standard cell potential () for the reaction below is . the cell potential for this reaction is when the concentration of and (s) + (aq) (s) + (aq) 0.78 1.10 0.94 1.26 1.42

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
The standard cell potential (e°cell) for the reaction below is +1.10v. the cell potential for this r...
Questions

Chemistry, 11.05.2020 05:57

English, 11.05.2020 05:57




Chemistry, 11.05.2020 05:57



Mathematics, 11.05.2020 06:57

Biology, 11.05.2020 06:57


Mathematics, 11.05.2020 06:57







Business, 11.05.2020 06:57

Chemistry, 11.05.2020 06:57

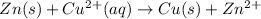
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Zn^{2+}]}{[Cu^{2+}]}](/tpl/images/0396/8274/2e6f5.png)
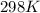
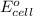 = standard electrode potential of the cell = +1.10 V
= standard electrode potential of the cell = +1.10 V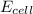 = emf of the cell = ?
= emf of the cell = ?