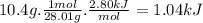The following thermochemical equation is for the reaction of sodium(s) with water(l) to form sodium hydroxide(aq) and hydrogen(g). 2na(s) + 2h2o(l)2naoh(aq) + h2(g) h = -369 kj when 6.97 grams of sodium(s) react with excess water(l), kj of energy are .
2) the following thermochemical equation is for the reaction of carbon monoxide(g) with water(l) to form carbon dioxide(g) and hydrogen(g).
co(g) + h2o(l)arrow. gif co2(g) + h2(g) delta16-1.gifh = 2.80 kj
when 10.4 grams of carbon monoxide(g) react with excess water(l), kj of energy are .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

You know the right answer?
The following thermochemical equation is for the reaction of sodium(s) with water(l) to form sodium...
Questions

English, 21.10.2019 21:30

History, 21.10.2019 21:30


Mathematics, 21.10.2019 21:30

Biology, 21.10.2019 21:30

Computers and Technology, 21.10.2019 21:30


Social Studies, 21.10.2019 21:30

Biology, 21.10.2019 21:30

Biology, 21.10.2019 21:30

English, 21.10.2019 21:30

Mathematics, 21.10.2019 21:30

Physics, 21.10.2019 21:30

Mathematics, 21.10.2019 21:30

Biology, 21.10.2019 21:30

Mathematics, 21.10.2019 21:30


English, 21.10.2019 21:30

History, 21.10.2019 21:30