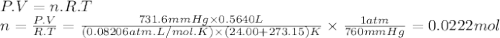Chemistry, 30.11.2019 03:31 fattypickeltoefungus
Oxygen gas can be prepared by heating potassium chlorate: 2kclo3(s)2kcl(s) + 3o2(g) in one experiment, a sample of kclo3 reacts and the gas produced is collected by water displacement. the gas sample has a temperature of 24.00 °c, a volume of 564.0 ml, and a pressure of 754.0 mm hg.
calculate the amount (in moles) of oxygen gas produced in the reaction. the vapor pressure of water is 22.38 mm hg at 24.00 °c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Oxygen gas can be prepared by heating potassium chlorate: 2kclo3(s)2kcl(s) + 3o2(g) in one experime...
Questions


Geography, 10.10.2019 19:50

Mathematics, 10.10.2019 19:50

Computers and Technology, 10.10.2019 19:50





Mathematics, 10.10.2019 19:50

History, 10.10.2019 19:50

Computers and Technology, 10.10.2019 19:50


History, 10.10.2019 20:00



Mathematics, 10.10.2019 20:00

Biology, 10.10.2019 20:00



History, 10.10.2019 20:00