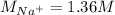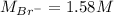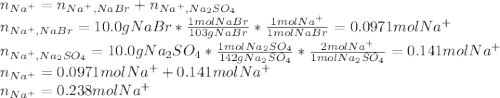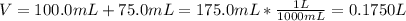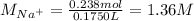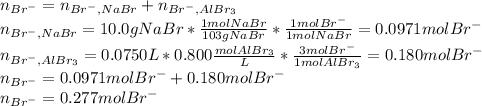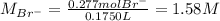Chemistry, 30.11.2019 03:31 caplode7497
Asolution is prepared by dissolving 10.0 g of nabr and 10.0 g of na2so4 in water to make a 100.0 ml solution. this solution is then mixed with 75.0 ml of a 0.800 m aqueous solution of albr3. calculate the concentration (m) of na+ and br− in the final solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
Asolution is prepared by dissolving 10.0 g of nabr and 10.0 g of na2so4 in water to make a 100.0 ml...
Questions

Social Studies, 03.02.2020 08:44


Mathematics, 03.02.2020 08:44



Physics, 03.02.2020 08:44

History, 03.02.2020 08:44

History, 03.02.2020 08:44

Mathematics, 03.02.2020 08:44



Mathematics, 03.02.2020 08:44


Biology, 03.02.2020 08:44



English, 03.02.2020 08:44

English, 03.02.2020 08:44

Mathematics, 03.02.2020 08:44

Mathematics, 03.02.2020 08:44