
Chemistry, 30.11.2019 03:31 emily200705
If the equilibrium constant for a reaction is 0.00010, what does this tell us about the position of equilibrium for that reaction?
a. there are more reactants than products at equilibrium.
b. there are more products than reactants at equilibrium.
c. at equilibrium, the concentration of reactants is the same as that of the products.
d. the concentration of the product at equilibrium is 0.00010 m.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

You know the right answer?
If the equilibrium constant for a reaction is 0.00010, what does this tell us about the position of...
Questions



History, 22.05.2020 11:00

Mathematics, 22.05.2020 11:00


Mathematics, 22.05.2020 11:00




Mathematics, 22.05.2020 11:00


Mathematics, 22.05.2020 11:00

English, 22.05.2020 11:00

Mathematics, 22.05.2020 11:00

Mathematics, 22.05.2020 11:00



Mathematics, 22.05.2020 11:00

Mathematics, 22.05.2020 11:00

Biology, 22.05.2020 11:00

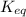
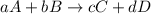
![K_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0396/7601/9c8b0.png)
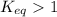 ; the reaction is product favored.
; the reaction is product favored.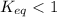 ; the reaction is reactant favored.
; the reaction is reactant favored.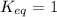 ; the reaction is in equilibrium.
; the reaction is in equilibrium.

