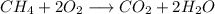Chemistry, 30.11.2019 04:31 wcraig1998
Arigid stainless steel chamber contains 140 torr of methane, ch4, and excess oxygen, o2, at 160.0 °c. a spark is ignited inside the chamber, completely combusting the methane. what is the change in total pressure within the chamber following the reaction? assume a constant temperature throughout the process.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
Arigid stainless steel chamber contains 140 torr of methane, ch4, and excess oxygen, o2, at 160.0 °c...
Questions

Mathematics, 22.10.2020 21:01



Biology, 22.10.2020 21:01

Mathematics, 22.10.2020 21:01

Social Studies, 22.10.2020 21:01


Social Studies, 22.10.2020 21:01


Mathematics, 22.10.2020 21:01

Mathematics, 22.10.2020 21:01

Mathematics, 22.10.2020 21:01


English, 22.10.2020 21:01

Social Studies, 22.10.2020 21:01

Mathematics, 22.10.2020 21:01


Mathematics, 22.10.2020 21:01