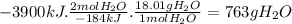Chemistry, 30.11.2019 05:31 HistoryLee
According to the following thermochemical equation, what mass of h2o (in g) must form in order to produce 3900 kj of energy?
sio2(s) + 4 hf(g) → sif4(g) + 2 h2o(l) δh°rxn = -184 kj sio2(s) + 4 hf(g) → sif4(g) + 2 h2o(l) δh°rxn = -184 kj 216 g 382 g 763 g 408 g 272 g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
How has the scientific community addressed the safety of chemicals? a. chemicals are repeatedly tested, even those that have existed for a long time. b. existing chemicals are tested if they have never been tested before. c. chemicals are tested if they are suspected to have caused a problem. d. only new chemicals are tested.
Answers: 2

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2
You know the right answer?
According to the following thermochemical equation, what mass of h2o (in g) must form in order to pr...
Questions

English, 28.12.2019 06:31




Geography, 28.12.2019 06:31

English, 28.12.2019 06:31

Mathematics, 28.12.2019 06:31

Spanish, 28.12.2019 06:31

English, 28.12.2019 06:31

Mathematics, 28.12.2019 06:31

Chemistry, 28.12.2019 06:31

Chemistry, 28.12.2019 06:31

Mathematics, 28.12.2019 06:31


History, 28.12.2019 06:31


Social Studies, 28.12.2019 06:31

Mathematics, 28.12.2019 06:31


Mathematics, 28.12.2019 06:31