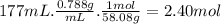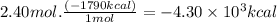Chemistry, 30.11.2019 05:31 gslovestodance6879
Consider the following thermochemical equation for the combustionof acetone, c3h6o, the main ingredient innail polish remover. c3h6o(l) + 4o2 (g) > 3co2 (g) + 3h2o (g), δhoof the reaction = -1790 kcalif a bottle of nail polish remover contains 177 ml of acetone, how much heat would be released by its complete combustion? thedensity of acetone is 0.788 g/ml.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
You know the right answer?
Consider the following thermochemical equation for the combustionof acetone, c3h6o, the main ingredi...
Questions


Biology, 10.02.2020 21:11



Mathematics, 10.02.2020 21:11

English, 10.02.2020 21:11


Mathematics, 10.02.2020 21:12



Mathematics, 10.02.2020 21:12

Mathematics, 10.02.2020 21:13

English, 10.02.2020 21:14


English, 10.02.2020 21:14



Mathematics, 10.02.2020 21:20