

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1
You know the right answer?
Calculate the amount of work done against an atmospheric pressure of 1.00 atm when 500.0 g of zinc d...
Questions




Mathematics, 23.07.2020 18:01









Mathematics, 23.07.2020 18:01

Mathematics, 23.07.2020 18:01






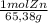 ×
×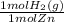 = 7,648 moles of H₂
= 7,648 moles of H₂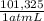 = 19,26 kJ
= 19,26 kJ

