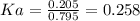Chemistry, 30.11.2019 05:31 lefthandeddolan
An acid-base indicator, hln, dissociates according to the following reaction in an aqueous solution. hinlag) in (aq) h (aq) the protonated form of the indicator, hln, has a molar absorptivity of 2929 m cm 1 and the deprotonated form, in has a molar absorptivity of 20060 m-1. cm 1 at 440 nm. the ph of a solution containing a mixture of hin and in s adjusted to 6.12. the total concentration of hin and in s 0.000127 m. the absorbance of this solution was measured at 440 nm in a 1.00 cm cuvette and was determined to be 0.818. calculate pka for hin.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1


Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
An acid-base indicator, hln, dissociates according to the following reaction in an aqueous solution....
Questions

Mathematics, 30.08.2021 17:50

Spanish, 30.08.2021 17:50

History, 30.08.2021 17:50





Social Studies, 30.08.2021 17:50



Mathematics, 30.08.2021 17:50


Mathematics, 30.08.2021 17:50


Mathematics, 30.08.2021 17:50





Mathematics, 30.08.2021 17:50

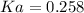
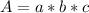
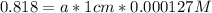
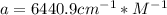
![x_{HIn]+x_{In}=1](/tpl/images/0397/0230/d5361.png)
![x_{HIn]=1-x_{In}](/tpl/images/0397/0230/0ee73.png)
![a=x_{In}*20060cm^{-1}*M^{-1}+ x_{HIn]*2929cm^{-1}*M^{-1}](/tpl/images/0397/0230/9addd.png)
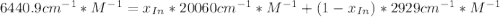
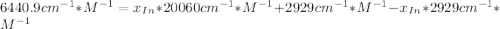
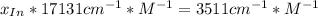
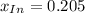
![x_{HIn]=1-0.205=0.795](/tpl/images/0397/0230/0ce5b.png)
![Ka=\frac{[In]}{[HIn]}](/tpl/images/0397/0230/065b2.png)