
Determine how many grams of each of the following solutes would be needed to make 3.40 × 102 ml of a 0.100 m solution. (a) cesium bromide (csbr): g (b) calcium sulfate (caso4): g (c) sodium phosphate (na3po4): g (d) lithium dichromate (li2cr2o7): g (e) potassium oxalate (k2c2o4): g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2


Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
Determine how many grams of each of the following solutes would be needed to make 3.40 × 102 ml of a...
Questions


Mathematics, 04.09.2020 05:01

Mathematics, 04.09.2020 05:01



Mathematics, 04.09.2020 05:01

English, 04.09.2020 05:01


French, 04.09.2020 05:01


Mathematics, 04.09.2020 05:01



Advanced Placement (AP), 04.09.2020 05:01

Mathematics, 04.09.2020 05:01




Mathematics, 04.09.2020 05:01

Physics, 04.09.2020 05:01

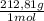 = 7,24g of CsBr -Where 212,81g/mol is molar mass of CsBr-
= 7,24g of CsBr -Where 212,81g/mol is molar mass of CsBr-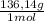 = 4,63g of CaSO₄
= 4,63g of CaSO₄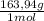 = 5,57g of Na₃PO₄
= 5,57g of Na₃PO₄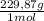 = 7,82g of Li₂Cr₂O₇
= 7,82g of Li₂Cr₂O₇ 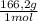 = 5,65g of K₂C₂O₄
= 5,65g of K₂C₂O₄

