
Chemistry, 30.11.2019 06:31 Virnalis1112
What is the [h3o+] and the ph of a benzoic acid-benzoate buffer that consists of 0.17 m c6h5cooh and 0.42 m c6h5coona? (ka of benzoic acid = 6.3 × 10^−5). be sure to report your answer to the correct number of significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2


Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3

Chemistry, 23.06.2019 23:00
My dog made become frightened and cover when he hears loud thunder this is an example of a reaction to what kind of stimuli a. chemicalsb. lightc. touchd. sound
Answers: 1
You know the right answer?
What is the [h3o+] and the ph of a benzoic acid-benzoate buffer that consists of 0.17 m c6h5cooh and...
Questions




















![[H_{3}O^{+}]=x M = 2.5\times 10^{-5}M](/tpl/images/0397/1268/ab31e.png) and pH = 4.6
and pH = 4.6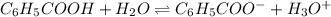
![\frac{[C_{6}H_{5}COO^{-}][H_{3}O^{+}]}{[C_{6}H_{5}COOH]}=K_{a}(C_{6}H_{5}COOH)](/tpl/images/0397/1268/75106.png)
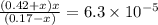
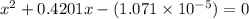
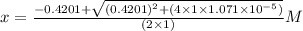
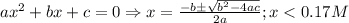 )
)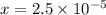 M
M![pH=-log[H_{3}O^{+}]=-logx=-log(2.5\times 10^{-5})=4.6](/tpl/images/0397/1268/f0ff1.png)



