
An element crystallizes in a face centered cubic lattice and has a density of 1.45 g cm-3 . the edge of its unit cell is 4.52 x 10-8cm.
a) how many atoms are in each unit cell?
b) what is the volume of a unit cell?
c) what is the mass of a unit cell?
d) calculate the approximate atomic mass of the element.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
An element crystallizes in a face centered cubic lattice and has a density of 1.45 g cm-3 . the edge...
Questions




Mathematics, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00



Mathematics, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00

Spanish, 11.11.2020 01:00

Advanced Placement (AP), 11.11.2020 01:00


Mathematics, 11.11.2020 01:00



Mathematics, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00


History, 11.11.2020 01:00

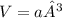 , a being the edge length.
, a being the edge length.

