
Chemistry, 30.11.2019 09:31 dudedude2201
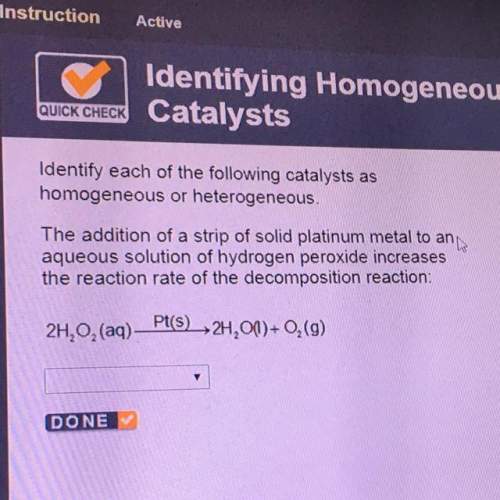
Identity each of the following catalysts as
homogeneous or heterogeneous.
the addition of a strip of solid platinum metal to an
aqueous solution of hydrogen peroxide increases
the reaction rate of the decomposition reaction

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
You know the right answer?
Identity each of the following catalysts as
homogeneous or heterogeneous.
the addition o...
homogeneous or heterogeneous.
the addition o...
Questions

English, 02.12.2021 01:00




English, 02.12.2021 01:00

Mathematics, 02.12.2021 01:00


Mathematics, 02.12.2021 01:00

History, 02.12.2021 01:00





Biology, 02.12.2021 01:00

History, 02.12.2021 01:00

Computers and Technology, 02.12.2021 01:00

Computers and Technology, 02.12.2021 01:00

Mathematics, 02.12.2021 01:00


English, 02.12.2021 01:00



