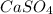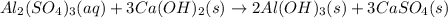Given the unbalanced equation:
- al(so )3 + - ca(oh)2 +_ al(oh)3 + caso,
what is the coef...

Chemistry, 01.12.2019 09:31 trinityparrish47
Given the unbalanced equation:
- al(so )3 + - ca(oh)2 +_ al(oh)3 + caso,
what is the coefficient in front of the caso, when the equation is completely balanced with
the smallest whole-number coefficients?
a. 1
b.2
c.3
d.4

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1
You know the right answer?
Questions



Mathematics, 26.05.2021 23:10

Social Studies, 26.05.2021 23:10

Mathematics, 26.05.2021 23:10

Social Studies, 26.05.2021 23:10

Arts, 26.05.2021 23:10





Mathematics, 26.05.2021 23:10





Mathematics, 26.05.2021 23:10

Mathematics, 26.05.2021 23:10