Chemistry, 02.12.2019 00:31 heatherswiffin666
Draw the structures for the following compounds:
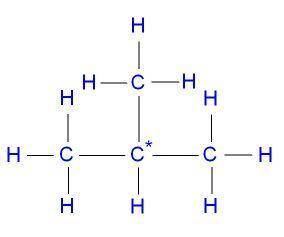
2-methylpropane

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
You know the right answer?
Draw the structures for the following compounds:
2-methylpropane...
2-methylpropane...
Questions


Mathematics, 12.02.2022 02:20


Mathematics, 12.02.2022 02:20

Mathematics, 12.02.2022 02:20



Physics, 12.02.2022 02:20

Social Studies, 12.02.2022 02:20

Mathematics, 12.02.2022 02:20


Chemistry, 12.02.2022 02:20

Biology, 12.02.2022 02:20


Mathematics, 12.02.2022 02:20




Biology, 12.02.2022 02:20

Mathematics, 12.02.2022 02:20