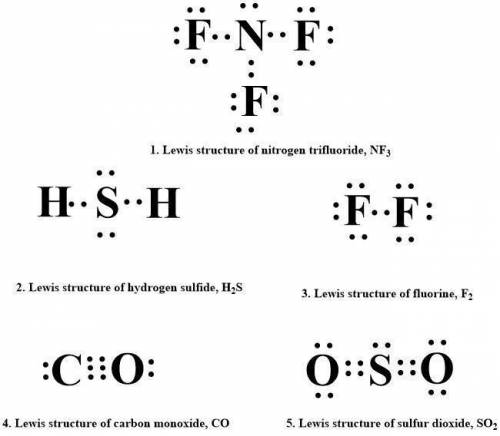
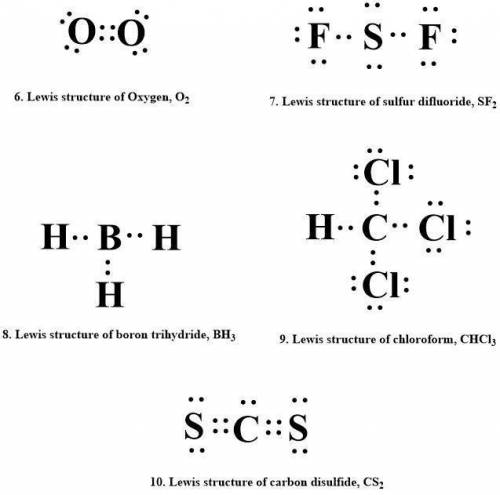
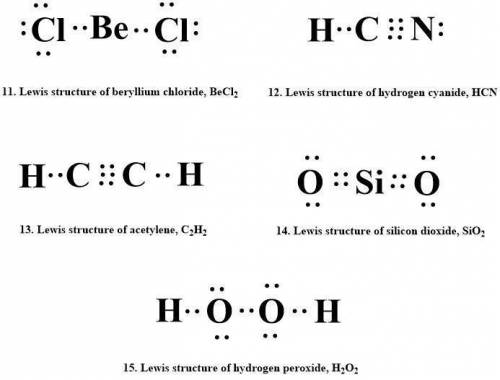
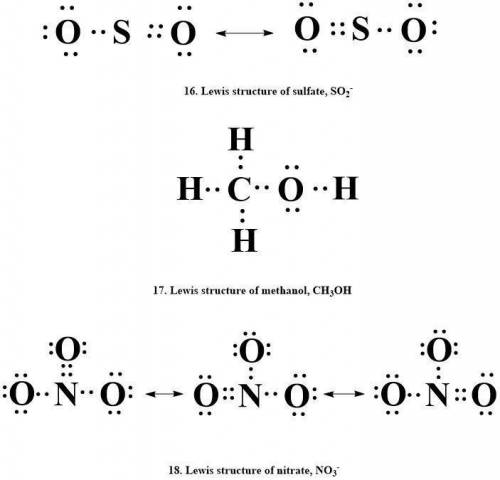
Draw lewis structures for each of the following.
1. nitrogen trifluoride, nf3
2. hydrogen sulfide, h2s
3. fluorine, f2
4. carbon monoxide, co
5. sulfur dioxide, so2
6. oxygen, o2
7. sulfur difluoride, sf2
8. boron trihydride, bhz
9. chloroform, chcl3
10. carbon disulfide, cs2
11. beryllium chloride, becl2
12. hydrogen cyanide, hcn
13. acetylene, c2h2
14. silicon dioxide, sio2
15. hydrogen peroxide, h2o2
16. sulfate, so2-
17. methanol, ch3oh
18. nitrate, no3
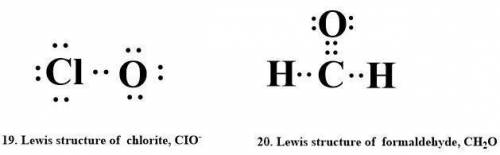
19. chlorite, cio,
20. formic acid, ch2o

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1


Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1
You know the right answer?
Draw lewis structures for each of the following.
1. nitrogen trifluoride, nf3
2. hydroge...
1. nitrogen trifluoride, nf3
2. hydroge...
Questions

Mathematics, 13.05.2021 23:40

Social Studies, 13.05.2021 23:40


Social Studies, 13.05.2021 23:40

Mathematics, 13.05.2021 23:40

English, 13.05.2021 23:50

Mathematics, 13.05.2021 23:50

Mathematics, 13.05.2021 23:50

History, 13.05.2021 23:50


Biology, 13.05.2021 23:50


Mathematics, 13.05.2021 23:50

Mathematics, 13.05.2021 23:50


English, 13.05.2021 23:50


Social Studies, 13.05.2021 23:50


Business, 13.05.2021 23:50