
Chemistry, 02.12.2019 19:31 elitehairnerd1964
When 100 ml of 1.0 m na3po4 is mixed with 100 ml of 1.0 m agno3,
a yellow precipitate forms and ag+ becomes negligibly small. which
of the following is the correct listing of the ions remaining in solution
in order of increasing concentration?
(a) po43- < no3- < na+
(b) po43- < na+ < no3-
(c) no3- < po43- < na+
(d) na+ < no3- < po43-
(e) na+ < po43- < no3-

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
When 100 ml of 1.0 m na3po4 is mixed with 100 ml of 1.0 m agno3,
a yellow precipitate fo...
a yellow precipitate fo...
Questions






History, 03.04.2020 22:28

Computers and Technology, 03.04.2020 22:28





Biology, 03.04.2020 22:28

Physics, 03.04.2020 22:28


Social Studies, 03.04.2020 22:28



Mathematics, 03.04.2020 22:28


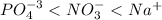
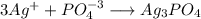
![[Ag^+]=0 M](/tpl/images/0399/5580/6ee64.png)
![[PO_4^{-3}]=0.5 M-0.5 M \frac{1 mol PO4}{3 mol Ag}=0.33 M](/tpl/images/0399/5580/8a590.png)
![[Na^+]=0.5 M * \frac{3 mol Na}{mol Na_3PO_4}=1.5 M](/tpl/images/0399/5580/07524.png)
![[NO_3^-]=0.5 M](/tpl/images/0399/5580/9ee3a.png)


