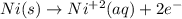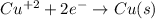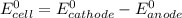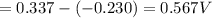Chemistry, 02.12.2019 21:31 loveashley1
Part a describe the electrodes in this nickel-copper galvanic cell. drag the appropriate items to their respective bins. view available hint(s) nickel copper standard reduction potentials for nickel(ii) and copper(ii) the standard reduction potential for a substance indicates how readily that substance gains electrons relative to other substances at standard conditions. the more positive the reduction potential, the more easily the substance gains electrons. consider the following: ni2+(aq)+2e−→ni(s),cu2+(aq)+2e−→cu( s), e∘red=−0.230 v e∘red=+0.337 v part b what is the standard potential, e∘cell, for this galvanic cell? use the given standard reduction potentials in your calculation as appropriate.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
You know the right answer?
Part a describe the electrodes in this nickel-copper galvanic cell. drag the appropriate items to th...
Questions

Advanced Placement (AP), 01.03.2021 22:20


Biology, 01.03.2021 22:20






Arts, 01.03.2021 22:20



Mathematics, 01.03.2021 22:20



Arts, 01.03.2021 22:20


Mathematics, 01.03.2021 22:20



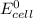 of the reaction is 0.567V.
of the reaction is 0.567V.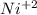 solution.
solution. 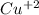 solution.
solution.