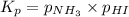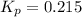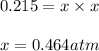Ammonium iodide dissociates reversibly to ammonia and hydrogen iodide. nh4i (s) ⇌ nh3 (g) + hi (g) at 400 ºc, kp = 0.215. calculate the partial pressure of ammonia at equilibrium when a sufficient quantity of ammonium iodide is heated to 400 ºc. complete the ice box below as part of your answer.
a. 0.103 atmb. 0.215 atmc. 0.232 atmd. 0.464 atme. 2.00 atm

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1


You know the right answer?
Ammonium iodide dissociates reversibly to ammonia and hydrogen iodide. nh4i (s) ⇌ nh3 (g) + hi (g) a...
Questions

English, 27.09.2019 21:30

Mathematics, 27.09.2019 21:30


History, 27.09.2019 21:30


English, 27.09.2019 21:30


Mathematics, 27.09.2019 21:30

History, 27.09.2019 21:30



History, 27.09.2019 21:30

Mathematics, 27.09.2019 21:30



Health, 27.09.2019 21:30


Health, 27.09.2019 21:30

English, 27.09.2019 21:30

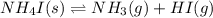
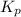 for the following equation is:
for the following equation is: