

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
Given thath2(g) + f2(g) -> 2hf(g) => ∆h = -546.6 kj . mol-12h2(g) + o2(g) -> 2h20(l) =&g...
Questions



Arts, 13.12.2021 18:10




Chemistry, 13.12.2021 18:10

Mathematics, 13.12.2021 18:10

Social Studies, 13.12.2021 18:10


Social Studies, 13.12.2021 18:10

Arts, 13.12.2021 18:10


Physics, 13.12.2021 18:10


Business, 13.12.2021 18:10


Mathematics, 13.12.2021 18:10


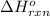 for the reaction is -521.6 kJ.
for the reaction is -521.6 kJ.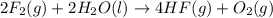
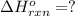
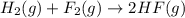
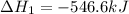 ( × 2)
( × 2)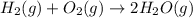
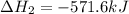
![\Delta H^o_{rxn}=[2\times \Delta H_1]+[1\times (-\Delta H_2)]](/tpl/images/0400/0579/648b7.png)
![\Delta H^o_{rxn}=[(2\times (-546.6))+(1\times (571.6))]=-521.6kJ](/tpl/images/0400/0579/83f77.png)


