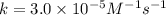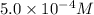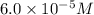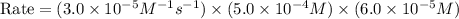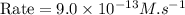Chemistry, 03.12.2019 01:31 PONBallfordM89
The reaction described by this equation
o3(g)+no(> o2(g)+no2(g)
has the following rate law at 310k.
rate of reaction=k[o3][no] k=3.0*10^6m^-1*s^-1
given that [o3]=5.0x10^-4m and no=6.0x10^-5m at t=0 calculate the rate of the reaction at t=0
what is the overall order of this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1


Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1

Chemistry, 23.06.2019 09:10
A155.0 9 piece of copper at 182 °c is dropped into 2500 g of water at 23.9 °c. (the specific heat of copper is 0.385 1/9°c.) calculate the final temperature of the mixture. (assume no heat loss to the surroundings.)
Answers: 2
You know the right answer?
The reaction described by this equation
o3(g)+no(> o2(g)+no2(g)
has th...
o3(g)+no(> o2(g)+no2(g)
has th...
Questions

History, 08.04.2020 19:00

History, 08.04.2020 19:00

Mathematics, 08.04.2020 19:00

Mathematics, 08.04.2020 19:00



English, 08.04.2020 19:00



Mathematics, 08.04.2020 19:00

Mathematics, 08.04.2020 19:00

Mathematics, 08.04.2020 19:00

Biology, 08.04.2020 19:00

History, 08.04.2020 19:00



Mathematics, 08.04.2020 19:00



Mathematics, 08.04.2020 19:01

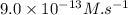

![\text{Rate}=k[A]^a[B]^b](/tpl/images/0400/1843/10aeb.png)
![[A]](/tpl/images/0400/1843/6aa06.png) and
and ![[B]](/tpl/images/0400/1843/db909.png) = concentration of A and B reactant
= concentration of A and B reactant
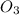 and
and 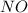 are the reactants.
are the reactants.![\text{Rate}=k[O_2][NO]](/tpl/images/0400/1843/1fdbe.png) ..........(1)
..........(1)